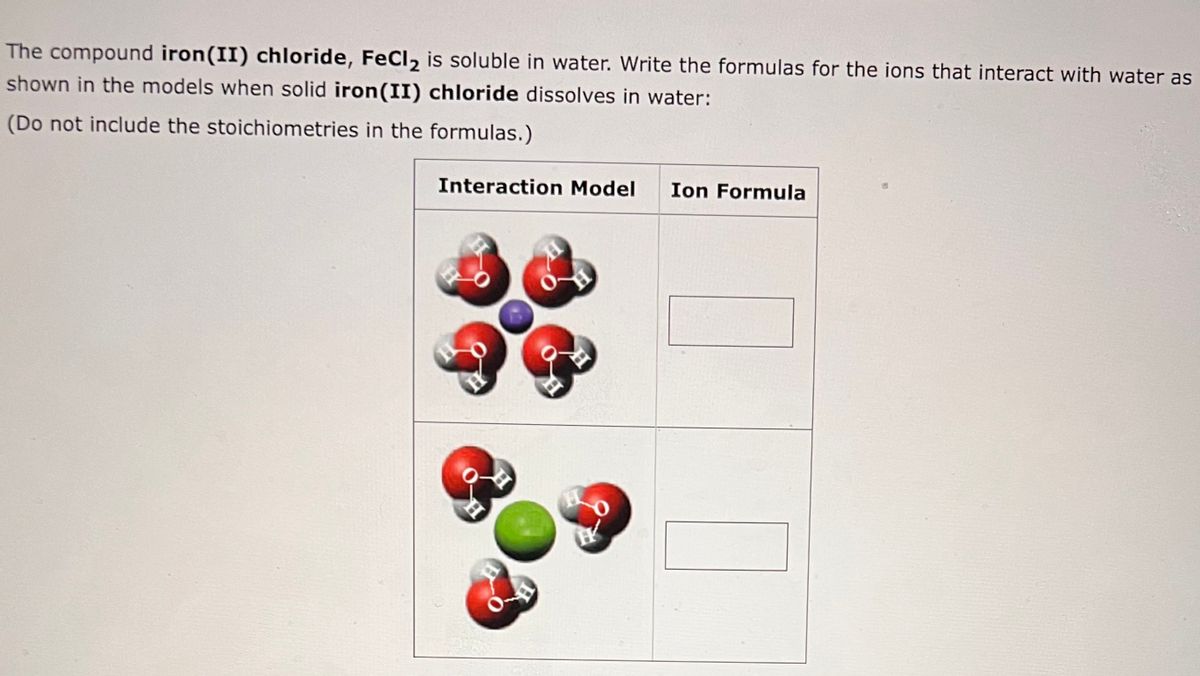
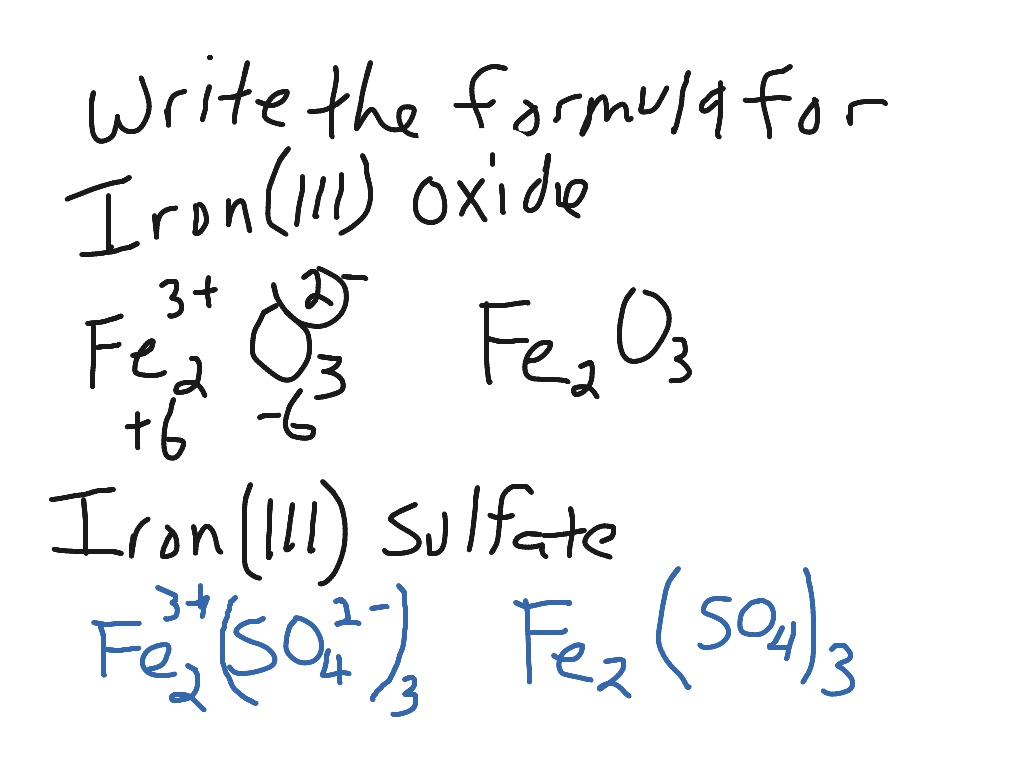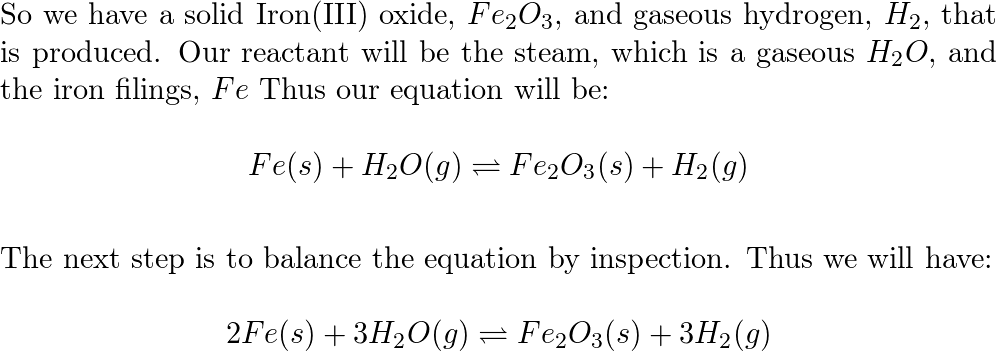Chemical Reactions”. All chemical reactions… have two parts: 1.Reactants = the stuff you start with 2.Products = the stuff you end up with The reactants. - ppt download
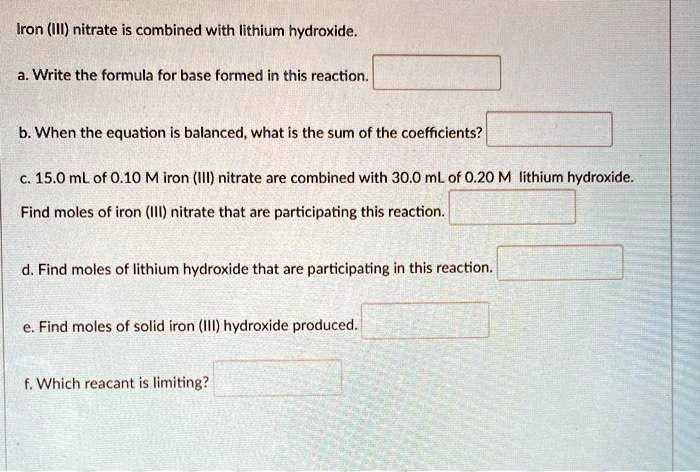
SOLVED: Iron (III) nitrate is combined with Iithium hydroxide. Write the formula for base formed in this reaction: b. When the equation is balanced, what is the sum of the coefficients? c.15.0

OneClass: Write the chemical equation for the dissolution reaction of solid iron(III) hydroxide in wa...

Question Video: Writing the Equation for 𝐾_𝑐 in the Reduction of Iron(II) Ions by Silver Ions | Nagwa